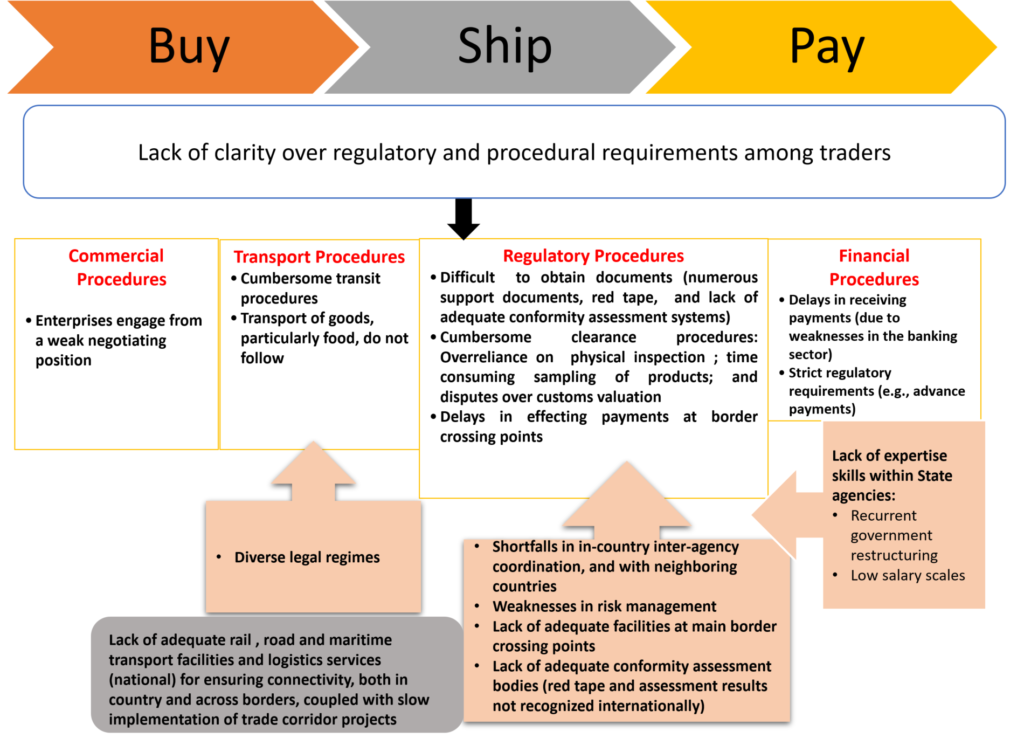
Common Challenges
Trade Facilitation
Quality Control & Assurance
Technical regulations and standardization
Absence of overall strategies for guiding the quality control and quality assurance function:
• The basic principles and building blocks for such a strategy are fragmented across an assortment of legislative acts and initiatives.
• Where available, strategiesare generic
• Technical regulations are not based on rigorous regulatory impact assessments


Conformity assessment bodies (CABs)
Conformity assessment results issued by national CABs are not recognized internationally
• Joining the European Cooperation for Accreditation (EA) Multilateral Recognition Arrangement (MLA) and International Laboratory Accreditation Cooperation (ILAC) mutual recognition agreement (MRA) remains a challenge. Not least because governments lack the resources to finance such key requirements as peer assessments and the translation of guides and technical documents.
• Market surveillance systems: Embryonic
Metrology
Calibration laboratories are not capable of demonstrating competence, measurement capability and traceability :
• In some countries legal metrology is not fully developed (Kyrgyzstan and Tajikistan)
• Calibration laboratories lack modern facilities
• Accreditation services are not covered by ILAC MRA.
• Calibration services are not covered by the Mutual Recognition Arrangement of the International Committee of Weights and Measures (CIPM MRA).


Implication
Ø- Calibration cannot be made in the international system of units (SI): traceability can only be established by the use of certified reference materials and inter-laboratory comparisons.Ø-Inter-laboratory comparisons comes with high costs that are beyond the financial resources available to national metrology bodies
